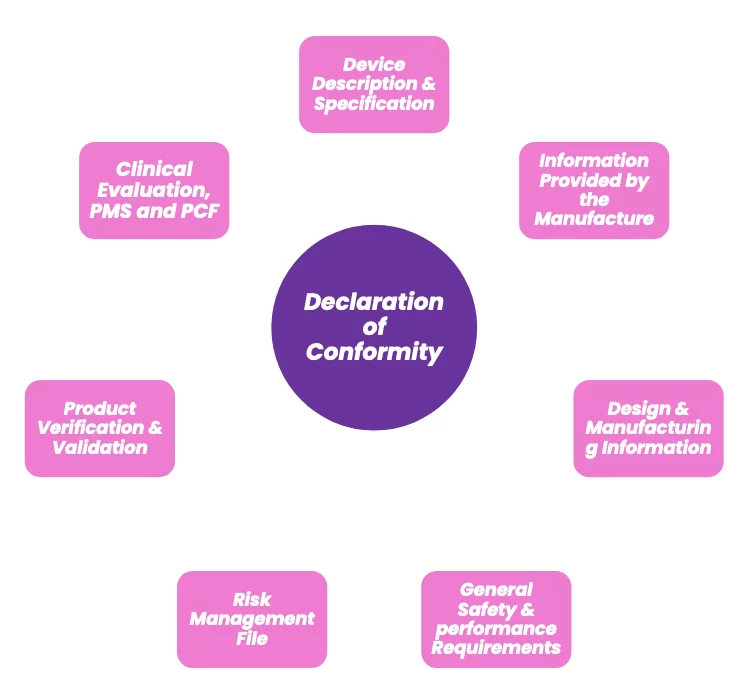
A technical file is required for medical devices marketed and sold in Europe and the UK.
Technical files are used to demonstrate a medical device complies with all applicable regulatory requirements and are reviewed during conformity assessments.
A product technical file should provide evidence of steps taken during the product lifecycle to meet safety and performance requirements. The technical file is a living set of documents which should be updated over the lifetime of the medical device and retained for 10 years post-market.
The file must demonstrate appropriate control, risk management, oversight, validation, and verification of the medical device and include design and development documentation.